Tue, Mar 4, 2025
[Archive]
Volume 21, Issue 4 (December 2024)
IJMSE 2024, 21(4): 61-68 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
rezayi Z, mirjalili M, Vahdati Khaki J. Effect of Mechanical Activation and Excess Graphite on the Formation of SrCO3 from Celestite Via Black-Ash Method. IJMSE 2024; 21 (4) :61-68
URL: http://ijmse.iust.ac.ir/article-1-3324-en.html
URL: http://ijmse.iust.ac.ir/article-1-3324-en.html
Abstract: (5096 Views)
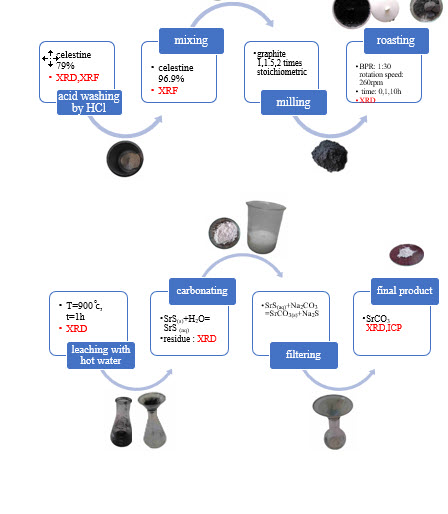
Celestite ore, the primary mineral for producing strontium compounds, particularly strontium carbonate, is processed using the black ash method, which involves carbothermic reduction, water leaching, and carbonation. This study aims to investigate the combined effect of mechanical activation and additional graphite on the recovery rate and purity of strontium carbonate. Celestite ore with a strontium sulfate content of 79% was obtained from the Dasht-e-Kavir mine. Acid washing with 10% hydrochloric acid significantly reduced carbonate impurities, resulting in a celestite purity of 96.9%. Mixtures of celestite and graphite with varying amounts of graphite were prepared with and without milling. The mixtures were roasted at 900 °C for 1 hour to form strontium sulfide, followed by hot water leaching. After filtration, sodium carbonate was added to the leachate containing SrS, resulting in the formation and precipitation of white strontium carbonate crystals. The results showed that the addition of graphite increased the recovery rate in unmilled specimens. However, the recovery rate decreased significantly when 1 and 10 hours of milling were applied in the presence of excess graphite. Conversely, in the absence of additional graphite, milling for 1 and 10 hours increased the strontium recovery rate to over 95%. Furthermore, the analysis of strontium carbonate obtained from the sample with the highest recovery rate showed a purity of over 99.9%.
Type of Study: Research Paper |
Subject:
Synthesis and preparation of materials to meet the requirements of AM techniques
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |