Tue, Dec 16, 2025
[Archive]
Volume 21, Issue 2 (June 2024)
IJMSE 2024, 21(2): 45-52 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Derakhshani M, rastegari S, Ghaffarinejad A. Ni-W Alloy Coating as an Efficient Electrocatalyst for the Hydrogen Evolution Reaction: Effect of Electroplating Current Density on Morphology and Electrocatalytic Properties. IJMSE 2024; 21 (2) :45-52
URL: http://ijmse.iust.ac.ir/article-1-3482-en.html
URL: http://ijmse.iust.ac.ir/article-1-3482-en.html
Abstract: (14821 Views)
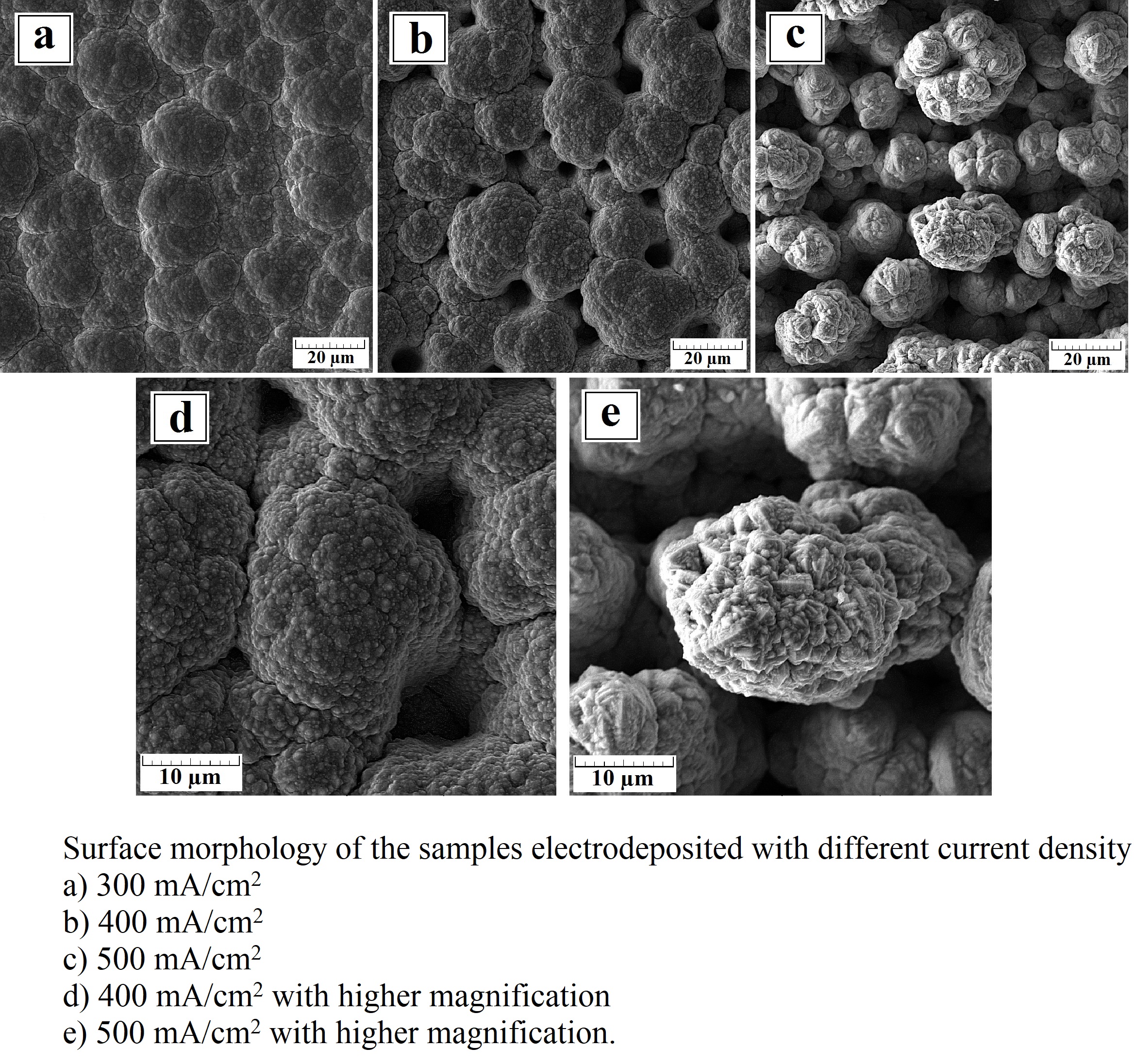
In this research, a nickel-tungsten coating as a catalyst for hydrogen evolution reaction (HER) with different current densities was synthesized and the resulting electrocatalytic properties and morphology were assessed. Linear sweep voltammetry (LSV), electrochemical impedance spectroscopy (EIS), and chronoamperometry in 1 M NaOH were used to evaluate the electrocatalytic activity for HER. By increasing the current density of electrodeposition up to 500 mA/cm2, a columnar morphology was observed. The cyclic voltammetry test (CV) revealed that when the plating current density increases, Cdl has increased from 248 to 1310 µF/cm2 and the active surface area increases 5 times. The results showed that by modifying the coating morphology, the current density of the hydrogen evolution increased up to two times.
Type of Study: Research Paper |
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |